Workshop 2005
Brain Mapping: From in vitro multisite recording to functional imaging.
BENEFRI Neuroscience Workshop 2005
March, 7th - 9th, Institute of Anatomy, University of Bern.
Organizers:
Thomas BERGER, Michele GIUGLIANO, Matthew LARKUM, René MüRI, Jürg STREIT
Speakers:
Thomas BERGER, Department of Physiology, University of Bern
Egidio D'ANGELO, Dept. of Physiological and Pharmacological Sciences, University of Pavia
Mathew E. DIAMOND, Cognitive Neuroscience Sector, SISSA, Trieste
Ulrich EGERT, Institute for Biology III, University of Freiburg / D
Olga GARASCHUK, Institute of Physiology, University of Munich
Michele GIUGLIANO, Department of Physiology, University of Bern
Alain KAELIN, Dept. of Neurology, University of Bern
Jason KERR, Max Planck Institute for Medical Research, Heidelberg
Thomas KöNIG, University of Bern
Matthew LARKUM, Department of Physiology, University of Bern
Shimon MAROM, Dept. of Physiology and Biophysics, Technion-Israel Institute of Technology, Haifa
René MüRI, Dept. of Neurology, University of Bern
Pavel OSTEN, Max Planck Institute for Medical Research, Heidelberg
Christoph OZDOBA, Dept. of Neuroradiology, University of Bern
Carl PETERSEN, Brain and Mind Institute EPFL Lausanne
Jürg STREIT, Department of Physiology, University of Bern
Roland WIEST, University of Bern
Participants of the BENEFRI Ph.D. Program in Neuroscience are requested to present a poster during the poster sessions describing their specific research projects.
Transportation charges are reimbursed to all participants of the BENEFRI Ph.D. program in Neuroscience.
No fees requested.
The workshop is also open for non-participants of the BENEFRI Ph.D. program in Neuroscience. Guests are welcome.
Contact: streit@pyl.unibe.ch
Final Program
Monday, March 7th
Extracellular multisite recording and stimulation
| 9:15 - 9:30 | Introduction J. Streit , Bern |
| 9:30 - 10:20 | Multisite recording in vitro with Multielectrode arrays (MEAs): technical background and applications. U. Egert, Freiburg |
| 10:50 - 11:40 | Rhythmic activity in cultured neural networks revealed by MEAs and patch clamp. J. Streit, Bern |
| 11:40 - 12:30 | Adaptation and learning in simple ex vivo networks. S. Marom, Haifa |
| Lunch | |
| 13:30 - 14:30 | Poster session 1 |
| 14:30 - 15:30 | The computer in the brain: Multisite and single cell recording in cerebellar slices. E. d'Angelo, Pavia |
| 15:30 - 16:30 | The function of cortical networks revealed by combining experimental with computational approaches. M. Giugliano, Bern |
| 17:00 - 18:00 | Watching the real brain: multisite recordings in vivo. M. E. Diamond, Trieste |
Tuesday, March 8th
Imaging techniques
| 9:15 - 9:45 | Introduction - Principles of Imaging Techniques (Thomas Berger - Department of Physiology Bern) |
| 9:45 - 10:30 | Influence of calcium channels on integration in cortical pyramidal cells (Matthew Larkum - Department of Physiology Bern) |
| 10:45 - 11:30 | Network activity studied in vitro with imaging techniques (Thomas Berger - Department of Physiology Bern) |
| 11:45 - 12:30 | Voltage-sensitive dye imaging in vitro and in vivo (Carl Petersen - EPFL Lausanne) |
| Lunch | |
| 13:30 - 14:30 | Poster session 2 |
| 14:30 - 15:15 | Imaging network activity in vivo (Olga Garaschuk - Institute of Physiology Munich) |
| 15:30 - 16:15 | Population calcium imaging reveals sparse heterogeneous neural activity in vivo (Jason Kerr - Max Planck Institute for Medical Research Heidelberg) |
| 16:30 - 17:15 | Lentiviral genetics: a novel approach to the study of neuronal functions in vivo (Pavel Osten - Max Planck Institute for Medical Research Heidelberg) |
| 17:30 - 18:00 | Final discussion |
Wednesday, March 9th
Clinical methods of brain mapping
| 9:15 - 10:45 | MR-mapping of the Brain: from morphology to function. Christoph Ozdoba, Bern |
| 11:00 -12:30 | Basal ganglia and deep brain stimulation in humans. Alain Kaelin, Bern |
| Lunch | |
| 13:30- 14:30 | Poster session 3 |
| 14:30 - 15:30 | Brain Mapping by TMS: from motor cortex to cognitive functions. René Müri, Bern |
| 15:45 - 16:15 | Neuroimaging of epilepsy: applied brain mapping. Roland Wiest, Bern |
| 16:30 - 18:00 | Spatial analysis of scalp recorded electric brain activity (EEG and ERPs) |
Poster Sessions Benefri Neuroscience workshop 2005
Poster Session 1, Monday, March 7th
Hao Ren, Institute for Infectious Diseases, University of Bern:
Experimental bacterial meningitis is associated with altered iron homeostasis in vulnerable brain regions: role of heme oxygenase?
Maura Arsiero, Department of Physiology, University of Bern:
The "Population-clamp" Technique: Linking Single-neuron Properties And Collective Network Activity In In Vitro Preparations
Cédric Yvon, Department of Physiology, University of Bern:
Use of riluzole, an INaP blocker, to investigate rhythm generation and intraburst oscillations in networks of spinal neurons
Julien Colomb, Institute of Zoology, University of Fribourg:
Different sensory projections define subregions in the first gustatory center of the drosophila larval brain
Poster Session 2, Tuesday, March 8th
Sarah Potez, Department of Physiology, University of Bern:
Ca-spike activity in the dendrites of layer 5 pyramidal neurons in vivo
Roland Schäfer, Department of Physiology, University of Bern:
Top-down modulation of V1 neurons in visual perception tasks
Christoph Lehmann, Dept. of Psychiatric Neurophysiology, University of Bern:
Hierarchical modulation of cross-modal interaction in human auditory cortex
Yihwa Kim, Department of Physiology, University of Bern:
The role of homeostasis in column formation
Maria Stein, Dept. of Psychiatric Neurophysiology, University of Bern:
Neurophysiological correlates of language learning: Preliminary results
Poster Session 3, Wednesday, March 9th
Miranka Wirth, Dept. of Psychiatric Neurophysiology, University of Bern:
Semantic processing in schizophrenia - preliminary results
Pascal Wurtz, Perception & Eye Movement Lab, DKF, University of Bern:
Inhibition of Return: Different time course in manual and saccadic response systems
Tobias Pflugshaupt, Perception & Eye Movement Lab, DKF, University of Bern:
The functional visual field: A new method to analyse outcomes of visual field defects
Susanne Jäggi, Institute of Psychology, University of Bern:
The cool brains of high-performers in situations of cognitive overload are even cooler: a dual-task investigation with fMRI at the edge of capacity
Alain Kaelin, Neurology Department, University of Bern:
Investigating motor units organization using a new mathematical model
Abstracts of oral presentations
Monday:
Multisite recording in vitro with Multielectrode arrays (MEAs): technical background and applications.
Uli Egert, Institute for Biology III, Freiburg/D
Although first reported in 1972, Microelectrode arrays have gained a more widespread use for extracellular recordings only recently. The lecture will cover the conceptual, technical and biophysical background for extracellular multi-electrode recordings and electrical stimulation in general. With a perspective on multi-site recordings in vitro from cell cultures and acute slices, approaches for the analysis, the interpretation and possible applications of the detected signal will be discussed.
Rhythmic activity in cultured neural networks revealed by MEAs and patch clamp
Jürg Streit and Pascal Darbon, Department of Physiology Bern
Rhythmic synchronous electrical activity of large populations of neurons are proposed to be related to many CNS functions like control of rhythmic movements, sleep, perception and even consciousness. In addition, during development, rhythmic activity is believed to be involved in the activity-dependent formation and refinement of networks. Rhythms can either be evoked by pacemaker neurons or emerge from network properties. We have investigated rhythm generation in cultured in vitro networks of foetal spinal cord cells using extracellular multisite recording by multielectrode arrays (MEAs) and intracellular recordings by patch clamp. In these networks reliable rhythm generation is induced by disinhibition. Such rhythms are based on repetitive bursts of network activation through glutamatergic recurrent excitation. These bursts spread from variable points (the burst sources) to the rest of the network. They are initiated by intrinsically firing neurons, which, in organotypic cultures, are mainly located around the central fissure of the spinal slices. Intrinsic spiking in these neurons is mainly based on a persistent Na current (INaP) and supported by an unspecific cation current, which is activated by hyperpolarization (Ih). When the neurons are pharmacologically disconnected from the network, intrinsic activity is usually tonic. However in the context of the network the neuronal activity is bursting in the same rhythm as the network. This finding is related to an activity-dependent auto-regulation of excitability of the neurons, which leads to a decrease in spike rate during the bursts and a network refractory period following the bursts. This auto-regulation is based on a slow inactivation of Na currents and an up-regulation of the Na/K pump. Together these findings show that the rhythm is not pacemaker-driven but emerges from properties of the network and its neurons. pdf
The computer in the brain: multisite and single cell recordings in cerebellar slices
D'Angelo E, Mapelli J, Dept. of Cellular and Molecular Physiological and Pharmacological Sciences University of Pavia and INFM, Via Forlanini 6, I-27100 Pavia, Italy (dangelo@ unipv.it)
The cerebellum has a complex network in which a couple of key neuronal types play critical computational roles. Although present knowledge on intrinsic excitability and synaptic transmission at individual network elements has progressed considerably, the spatial distribution of electrical signals remains largely unknown. This is a critical aspect indeed, since the cerebellar cortex is thought to operate a complex spatio-temporal transformation of incoming signals. We have performed multi-electrode recordings from acute cerebellar slices obtained from P18-P24 rats at 30±1 °C. The recording system (MEA60, Multichannel Systems) allows simultaneous acquisition from 60 electrodes spaced by 100 ?m. Spontaneous activity appeared as simple and complex spikes in Purkinje cells and as autorhythmic firing in Golgi cells. No spontaneous activity related to granule cells could be detected. Granular layer activity was evoked by electrical stimulation of the mossy fibre bundle, and the stimulus artefact was subtracted by using tracings recorded after 1 ?M TTX application at the end of the experiment. In the presence of the GABA-A receptor blocker 10 ?M bicuculline, the waveform showed the typical P1-N1-N2 sequence corresponding to mossy fibre and granule cell excitation. N2 was inhibited by 10 ?M NBQX and 25 ?M APV, indicating that they were mostly determined by AMPA and NMDA receptor activation. The N2 delay increased with distance, yielding a diffusion velocity of ~0.1 m/sec. When bicuculline was omitted, PC discharge increased and a large repolarising wave in the granular layer (P2) signalled enhanced PC excitation following GrC activity. Application of theta-burst stimulation (TBS) caused LTP and LTD in different electrodes. Bicuculline broadened and increased excitation and biased plasticity toward LTP. Subtraction experiments showed that LTP was most likely to occur on the electrodes that were initially less inhibited. Moreover, LTP was correlated with short N2 delays, indicating that stronger excitation favoured LTP. These results are revealing that LTP and LTD are spatially controlled by the intensity of excitation and inhibition in the cerebellar network, providing the basis for granular layer plastic re-mapping following the arrival of appropriate discharge through the mossy fibre pathway.
Watching the real brain: multisite recordings in vivo
Mathew E. Diamond, Cognitive Neuroscience Sector, SISSA, Trieste
(1) Why multisite recordings in vivo are important
(2) Overview of a few of the main methods
(3) Evidence for brain function obtained by multisite recordings in vivo: a brief survey
(4) Our evidence: Plasticity of horizontal connections in rat "barrel" cortex
Suggested readings: pdf1, pdf2
Tuesday:
Influence of calcium channels on integration in cortical pyramidal cells
Matthew Larkum - Department of Physiology Bern
The pyramidal cell is the principal excitatory neuron of the neocortex. It is the major cell type with long range axonal projections to other cortical areas and out of the cortex altogether. Thus the pattern of action potentials in a population of pyramidal neurons must encapsulate the information to be transmitted. It is therefore vital to understand how this cell type converts input to output. The most distinctive thing about pyramidal neurons is their morphology. An apical dendrite, often more than 1 mm, extends from towards the top layers of the cortex where it branches profusely in an area known as the tuft. This morphology allows the pyramidal neuron to receive inputs from multiple layers while simultaneously presenting the problem that many synaptic inputs make contacts at great distances from the cell body. In recent years, a great amount of study has been devoted to exploring this problem. It has become clear that voltage-gated calcium channels and calcium released from intracellular stores play a great role in affecting the properties of pyramidal neurons. Much of this work has benefited from the use of calcium imaging of pyramidal neurons in the cortex.
I will show experiments from my own work focussing on the imaging of calcium spikes in the tufts of pyramidal neurons and the propagation of waves of calcium
Network activity studied in vitro with imaging techniques.
Thomas Berger - Department of Physiology Bern
The isolated use of calcium- and voltage-sensitive dyes (CaSDs and VSDs), respectively, offers great possibilities to study neuronal activity in neuronal networks. Aside from some technical drawbacks of these dyes, imaging with one of these dyes gives us, however, only the tools to study certain aspects of network activity: Either primarily superthreshold activity with CaSDs or nearly exclusively subthreshold postsynaptic signals using VSDs. We have developed a methodology for the use of both dye types in whole cortical columns of the somatosensory barrel cortex. While CaSDs can be used to monitor primarily presynaptic action potential activity, postsynaptic sub-threshold signals are nearly exclusively detected with VSDs. The combined use of VSDs and CaSDs will join the advantages and circumvent at least part of the disadvantages for the study of network activity.
Voltage-sensitive dye imaging in vitro and in vivo.
Carl Petersen - EPFL Lausanne
Voltage-sensitive dye imaging in vivo allows the millisecond resolution of subthreshold membrane potential changes in the supragranular layers of the cortex with a lateral resolution of approximately 50 microns. This technique allows real time imaging of the sensory response in mouse barrel cortex evoked by a whisker stimulus. Using flexible fiber optic bundles we have now been able to image cortical function with voltage-sensitive dyes in awake and freely moving mice. By simultaneously filming the behavior of the animal we can match at up to 2ms resolution on a frame-by-frame basis the whisker-related behavior of the mouse and the spatiotemporal dynamics of primary somatosensory barrel cortex. These experiments give us the first high resolution images of sensory processing in awake free moving mice.
Imaging network activity in vivo.
Olga Garaschuk, Helmut Adelsberger and Arthur Konnerth, Institute of Physiology, Ludwig-Maximilian University Munich, Germany
Although neuronal networks play a key role in information processing, many of their functional properties in vivo are unknown. For a direct in vivo monitoring of network function, cells in defined cortical regions were stained with a membrane-permeant calcium indicator dye (e.g. Calcium Green-1 AM), delivered locally from a micropipette. The activity-dependent changes in the intracellular Ca2+ concentration were monitored either at a single cell resolution using two-photon microscopy or at the population level using an implanted optical fiber (Ø 100-200 µm, NA 0.48).
In newborn mice this approach revealed a specific form of large-scale spontaneous calcium waves, which occurred at a frequency of 3-10 per minute and lasted for 3-5 s. These Ca2+ waves were observed only in non-anesthetized animals and were reversibly blocked by anesthetics like isoflurane. A combined in vivo/in vitro analysis performed in the same brains showed that the waves have neuronal origin (involving 95% of cortical neurons), require action potential firing as well as glutamatergic synaptic transmission and spread with a mean speed of 7 mm/s. Remarkably, the Ca2+ waves were detected only during the periodically occurring resting states that are characteristic for early stages of postnatal life. Thus, the described combination of calcium recordings techniques allows to monitor activity of neuronal networks in vivo in anaesthetized as well as behaving animals providing new insights into their functional properties.
Population calcium imaging reveals sparse heterogeneous neural activity in vivo.
Jason N. D. Kerr and Fritjof Helmchen, Dept. Cell Physiology, Max-Planck-Institute for Medical Research, Heidelberg, Germany
Understanding the principles of information processing in neural networks requires the measurement of spatiotemporal patterns of electrical activity in cell populations under in vivo conditions. The combination of in vivo two-photon microscopy and recent advances in labelling techniques now permits fluorescence measurements from calcium-indicator loaded cell populations in the neocortex of anesthetized animals. Here we demonstrate how these optical measurements can be combined with electrophysiological recordings to reveal spatiotemporal patterns in cortical networks, in vivo. Neocortical cell populations were labelled using bolus-injection through a patch pipette containing AM-ester calcium indicator, Oregon Green BAPTA-1. Astrocytes were in addition counterstained using the red fluorescent dye sulforhodamine 101. Because the calcium dye not only stains cell bodies but also dendrites and axons and boutons, a prominent diffuse signal component was present in the neuropil. We found that this neuropil signal shows strong fluctuations that are highly synchronized with the ongoing spontaneous activity (up- and down-states) in the neocortical network as measured both with an EEG electrode and using whole-cell recordings. Isolating the pre- and postsynaptic components of the neuropil signal revealed that this 'optical encephalogram recording' (OEG) is of presynaptic origin, thus representing the input activity in a cortical area. In addition, single action-potential evoked somatic calcium transients were detected from targeted cell-attached recordings from labelled neuronal somata. Together this allowed optical measuring of presynaptic barrages that represent local input to this area and spiking patterns in a small group of neurons, representing the cortical output activity. We used this method to investigate spatiotemporal activation patterns during spontaneous cortical Up-states and show sparse heterogeneous neuronal output activity and its relation to presynaptic input.
Lentiviral genetics: a novel approach to the study of neuronal functions in vivo.
Pavel Osten, Max Planck Institute for Medical Research, Heidelberg, Germany
Genetic manipulations, such as gene knock-out and transgenic techniques, provide an invaluable tool for studying molecular mechanisms underlying neuronal functions at the system level. Here I will present a method which can be used for studying individual genetically altered neurons in otherwise intact brain, thus providing a novel tool for analysis of neuronal functions at the cellular level in vivo. We use lentiviral vectors to stably infect cortical neurons in rats and mice, by stereotaxically-aided delivery of the viral particles to selected cortical subregions. We have developed several vectors which can be used for: 1. neurospecific transgenic expression; 2. siRNA-based gene silencing; and 3. Cre recombinase-based gene knock-out. The main advantages of this approach lie in the ease of its application and in the temporal and spatial control of the genetic manipulations. Furthermore, the cellular phenotype of the genetic manipulations can be assessed either in vitro in acute cortical slices or in vivo by two-photon microscopy targeted whole-cell recordings. In this technique, two-photon imaging is used to simultaneously visualize a whole-cell pipette containing a fluorescent Alexa-dye and EGFP fluorescent neurons, which allows us to guide the patch pipette toward the labelled cells.
Wednesday:
MR-Mapping of the Brain: From Morphology to Function
Christoph Ozdoba, Institute of Diagnostical and Interventional Neuroradiology, University of Bern
Since its introduction into clinical routine in the early to mid-eighties, magnetic resonance imaging (MRI) has become the imaging method of choice for most neuroscience issues in humans. MRI acquires sectional images without use of ionizing radiation. Though contraindications exist and certain safety regulations have to be obeyed, the method is widely usable in all patients, even newborns (or unborn children). As MRI is based on chemical properties of the tissue that is examined, image contrast can be varied by changing certain parameters. Contrary to computed tomography (CT) that is limited to the acquisition of images in the axial plane, i.e., perpendicular to the body's long axis, MRI allows to acquire images in any spatial orientation. Its excellent soft tissue contrast is superior to CT, furthermore, image contrast is not degraded by bony tissue.
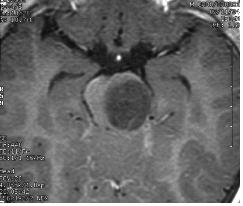
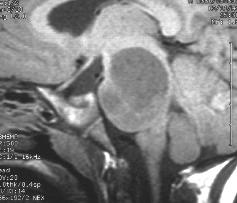
Left and center: Excellent tissue contrast allows to clearly delineate this low grade brainstem tumour in a child.
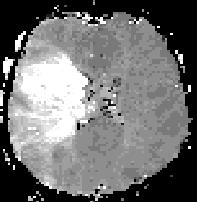
Right: Perfusion imaging shows hypoperfusion in the vascular territory of the right middle cerebral artery in a patient with acute ischemic stroke
Since the early 1990s, MRI has gone beyond mere morphological (anatomical) imaging into new fields summarized as "functional imaging." This includes techniques like diffusion weighted imaging, perfusion imaging, or the mapping of cortical activation, i.e., it goes ahead of morphology to the actual measurement and visualization of physiological processes in the brain. In these fields, clinical applications are catching up, but they still play a minor role in comparison to basic research in the realm of neurophysiology and neuropsychology.
Neuroimaging of epilepsy: applied brain mapping
Roland Wiest, Institute of Diagnostical and Interventional Neuroradiology, University of Bern
During the last decade there has been a dramatic increase in the number of surgical procedures being performed for medically refractory partial epilepsy. Patients with focal epilepsy are potential candidates for epileptic surgery, if an epileptogenic focus can be delineated conclusively. Advances in the delineation of epileptogenic foci have come from an increasing number of improved imaging techniques in the field of MRI, SPECT and PET, and through the development of software to allow co-registration of different investigative modalities. The application of combined and observer independent imaging techniques is of particular interest in refractory temporal epilepsy and extratemporal epilepsy syndromes. However, even in the presence of structural abnormalities, the extent and location of an epileptogenic area cannot be clearly depicted from anatomical information alone. Beyond detailed clinical evaluation and video-EEG analysis, newly developed imaging methods as diffusion tensor imaging (DTI), chemical shift imaging, voxel-based 3-D MRI analysis, EEG-correlated functional MRI and ictal subtraction SPECT and MRI coregistration techniques offer the opportunity to localise epileptogenic foci properly. Typical structural neuroimaging findings will be discussed, followed by an overview on newer imaging techniques in the field of epileptology.
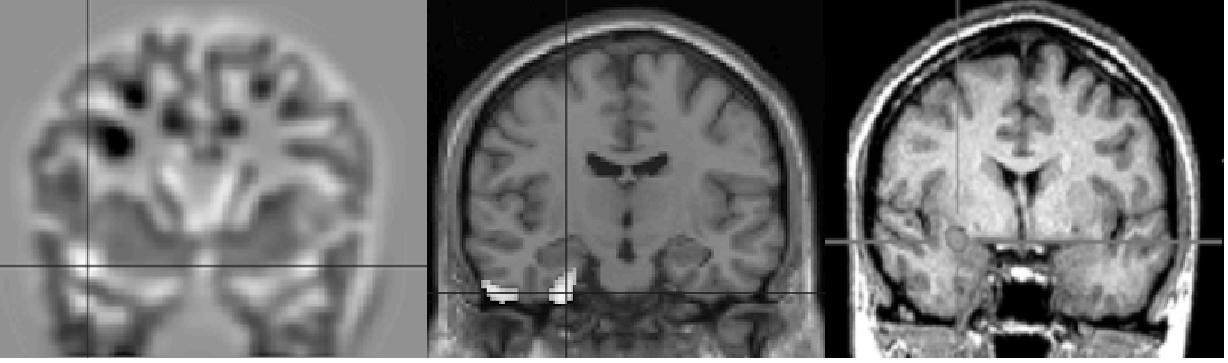
| Voxel-based 3-D MRI | FDG-PET | SISCOM |
|---|
Different imaging techniques for the delineation of an epileptogenic focus in temporal lobe epilepsy: Voxel-based 3-D MRI analysis of the anatomical T1w-MRI, FDG-PET and subtraction SPECT co-registered to MRI (SISCOM) reveal different characteristics of the epileptogenic lesion: Additional cortical abnomalitiy and hypometabolism beyond the mesial temporal lobe (left and center image) and ictal hyperperfusion of the epileptogenic zone (left image)
Spatial analysis of scalp recorded electric brain activity (EEG and ERPs)
Thomas Koenig - Department of Psychiatric Neurophysiology, University Hospital of Clinical Psychiatry Bern
Intracerebral electric activity with sufficient common orientation and of sufficient strength produces measurable, widespread potential differences on the scalp; these are called EEG (electroencephalogram). The physics that determine, - from a given set of active sources in the brain -, amplitude and spatial distribution (i.e. the EEG topography) of the scalp measurements is well known: A given set of sources will always produce the same, predicable EEG topography. Changes in EEG topography thus always indicate changes of active sources and thus assumingly changes in the functional state of the brain. The comparison of EEG topographies can therefore be used to track changes of brain functions over time, subjects or experimental conditions. Several such examples and some implications of this approach will be given.
When stimuli are given during an EEG recording and the EEG is averaged time-locked to these events (i.e. so-called ERPs, event-related potentials are computed), one can study the sequence of brain states associated with the processing of the given stimuli. This is namely interesting for the study of unconscious or pre-conscious processing, or for a comparison of processing strategies in different subject groups.
The localization of the sources of a measured EEG/ERP topography is a problem that does not have a unique solution, since different source configurations may produce the same topography. However, one can make reasonable a-priori assumptions about the configuration of active sources and obtain physiologically plausible hypotheses about intracerebral current source densities under different conditions. Several source localization approaches will be introduced and exemplified.
This workshop is supported by Roche, Novartis and Serono
 |
 |
 |


